Anorexia nervosa is not merely a dietary choice; it is a complex and severe mental health disorder that manifests through restrictive eating, an intense fear of gaining weight, and pervasive distortions in body image. Often overlooked, this condition can exacerbate anxiety and depression while leading to potentially life-threatening malnutrition. As researchers delve deeper into the complexities of anorexia, recent studies have uncovered significant links between alterations in brain chemistry and the onset of this debilitating condition. Such findings promise to enhance our understanding of anorexia and may pave the way for improved therapeutic strategies.
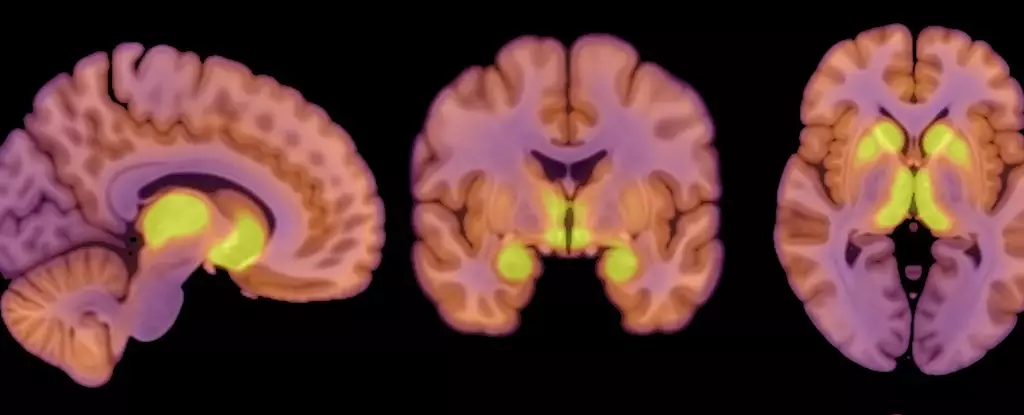
Research suggests that anorexia nervosa may stem, at least in part, from changes in neurotransmitter functions, particularly those concerning the brain’s reward systems. Previous investigations have established a correlation between anorexia and notable alterations in brain structure, highlighting the deficient neurotransmitter acetylcholine’s role in the striatum—an area crucial for motivation and reward processing. Within this context, a recent study has brought to light the significance of mu-opioid receptors (MORs), integral players in the brain’s extensive opioid system, which modulates both eating behaviors and the sensations of pleasure associated with food.
Researchers, including physiologist Pirjo Nuutila from the University of Turku, assert that increased MOR availability in specific brain regions correlates strongly with anorexia. This elevation in MOR levels suggests that individuals with anorexia may experience heightened neurochemical impulses that influence their appetite and satisfaction derived from food. This is in stark contrast to findings in individuals with obesity, where opioid system activity typically diminishes. The distinctions drawn here are vital for future therapeutic approaches, potentially aiming to recalibrate these neurochemical pathways in patients suffering from anorexia.
The recent study involved a sample group of 13 women diagnosed with anorexia nervosa, whose ages ranged from 18 to 32 and whose body mass index (BMI) fell below 17.5 kg/m². These patients were contrasted with a control group of 13 healthy females of similar age but with BMIs between 20 and 25 kg/m². Utilizing positron emission tomography (PET) scans, the research team meticulously measured both MOR availability and brain glucose uptake, aiming to assess how these metrics correlate with the restricted caloric intake typical of anorexia patients.
Interestingly, the researchers found that the brains of those suffering from anorexia demonstrated glucose uptake comparable to healthy controls, even amidst their critical energy deficits. This finding indicates that the brain prioritizes its energy needs, attempting to maintain functionality even when overall body nourishment is insufficient. Co-author Lauri Nummenmaa commented on this, noting that it illustrates a potential protective mechanism of the brain against severe undernutrition.
The juxtaposition of elevated MOR availability with stable glucose uptake presents a compelling narrative about the neurobiological underpinnings of anorexia. This study posits that the body’s opioid system could play a pivotal role in the disorder’s persistence, reflecting a possible neurochemical “feedback loop” that both sustains and exacerbates the condition. As weight is lost, the body’s up-regulated MOR system may contribute to the continuous restriction of food intake, creating a vicious cycle that can be challenging to break.
However, the researchers caution about the limitations of this study. The all-female sample size limits the applicability of these results across genders, as anorexia can affect individuals regardless of sex. Moreover, the small cohort, consisting of only 26 participants, underscores the need for further studies to validate and expand upon these findings. By foregoing subjective questionnaires due to the sensitivity of eating behaviors in those with anorexia, the researchers also acknowledged the potential gap in directly linking MOR changes to patient-specific dietary practices.
Despite its limitations, this study underscores a significant connection between neurobiological factors and anorexia nervosa. As researchers like Nummenmaa elucidate, fluctuations in brain function relate closely to both appetite regulation and overall body weight adjustments. Enhanced understanding of how these mechanisms interact within the brain can open new avenues for targeted treatments that address both the physiological and psychological dimensions of the disorder.
While anorexia nervosa remains a challenge in mental health, recent research contributes invaluable insights into its underlying biological processes. Such knowledge is instrumental not only for fostering better treatment modalities but also for offering hope to those grappling with this complex and often tragic condition. As we continue to explore this mental health crisis, the interplay of brain chemistry and behavior will undoubtedly reveal more about the intricate tapestry of anorexia nervosa.