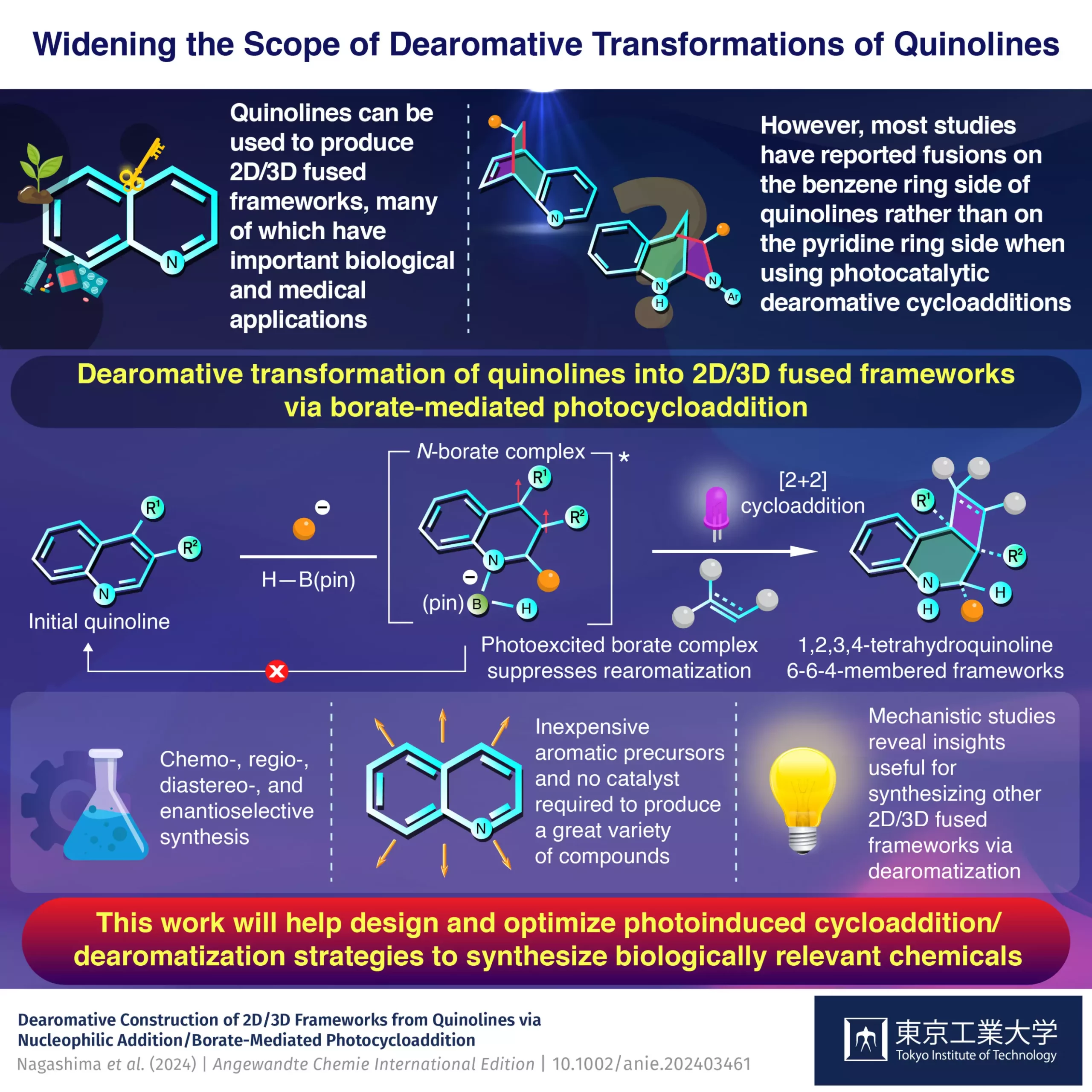
The realm of synthetic organic chemistry has always been characterized by its quest for innovative methodologies that propel the field forward. Recently, researchers from Tokyo Institute of Technology, led by Assistant Professor Yuki Nagashima, have made significant strides by employing quinoline derivatives as precursors for the synthesis of complex 2D/3D fused frameworks. This ground-breaking approach not only utilizes affordable starting materials but also leverages light-activated processes to enhance the yield and variety of resultant compounds—a promising avenue for drug development.
Quinolines, organic compounds known for their unique structural features, have long piqued the interest of chemists due to their dual electronic nature. They comprise an electron-rich benzene ring fused with an electron-deficient pyridine ring, allowing for selective modifications. Until now, synthesis efforts predominantly targeted the benzene portion, limiting the exploration of the more reactive pyridine side. Nagashima’s team recognized this gap as an opportunity to harness the full potential of quinolines in creating diverse frameworks with substantial pharmaceutical applications.
A Light-Empowered Synthesis Strategy
At the heart of this innovative synthesis strategy lies a method known as dearomative photocycloaddition. Here, researchers employ pinacolborane (H-B(pin))—a boron-centered compound—as a catalyst. What is particularly exciting about this methodology is its ability to shift the focus toward the pyridine side of quinoline, leading to the generation of previously inaccessible 2D/3D structures. The atmospheric stability of quinolines combined with the light-induced reactivity offers a perfect playground for creating complex yet customizable compounds vital in drug discovery.
The instrumental role played by the borate intermediate formed during the initial reaction is critical. Nagashima’s research indicates that this borate complex not only facilitates the cycloaddition but also inhibits rearomatization— a common pitfall in traditional synthetic routes. By redefining the processes at play, the team effectively bypasses many complications typically associated with quinoline chemistry, leading to higher yields of desired products.
Significant Practical Advantages
One of the most serious hurdles in traditional organic synthesis remains the reliance on costly and arduous multi-step processes accompanied by the use of catalysts. Nagashima’s methodology stands out by significantly reducing the complexity of the procedure—fewer steps and shorter reaction times translate directly to enhanced efficiency and cost-effectiveness. Additionally, the use of multi-substituted quinoline derivatives expands the variety of potential target compounds, which fuels innovative applications across various scientific disciplines, particularly in medicinal chemistry.
Furthermore, the absence of catalysts not only lowers operational costs but also minimizes contaminant introduction into products, rendering the resultant compounds cleaner and more amenable to application. This streamlined process could revolutionize how synthetic chemists approach the creation of complex organic molecules, fundamentally changing the landscape of pharmaceutical development.
Real-World Implications of New Discoveries
The implications of this research extend beyond academic curiosity; they promise real-world impact in drug development. With an ability to tailor the structure of compounds at a molecular level, this synthesis strategy provides the tools for producing highly customizable drug candidates. Given that many therapeutic agents rely on specific molecular configurations for their efficacy, having access to a variety of fused frameworks could significantly enhance the design process of new pharmaceuticals.
Moreover, the insights gleaned from the underlying mechanisms of this innovative approach pave the way for future research endeavors. By offering a pathway to access and explore additional boron-containing compounds, scientists can further innovate in the synthesis of multi-ringed aromatic hydrocarbons— a vast area with numerous applications in material science and drug development.
The research led by Nagashima and his team offers a fresh perspective on quinoline utilization that could significantly alter the methodology landscape in organic chemistry. Their findings not only showcase the value of inexpensive starting materials in creating complex frameworks but also reflect a broader trend of focusing on efficiency, efficacy, and economic viability in chemical synthesis. The future appears bright for quinoline chemistry, with vast potential waiting to be unlocked.