The electrochemical reduction of carbon dioxide (CO2) presents a beacon of hope in addressing climate change and promoting sustainable chemistry. As we grapple with rising CO2 levels in the atmosphere, the conversion of this greenhouse gas into valuable products is pivotal. Historically, the focus has mainly been on optimizing catalysts to enhance the efficiency and selectivity of this process. However, recent advancements suggest that electrolyte composition could significantly influence the trajectories of these reactions, offering researchers a new avenue for exploration.
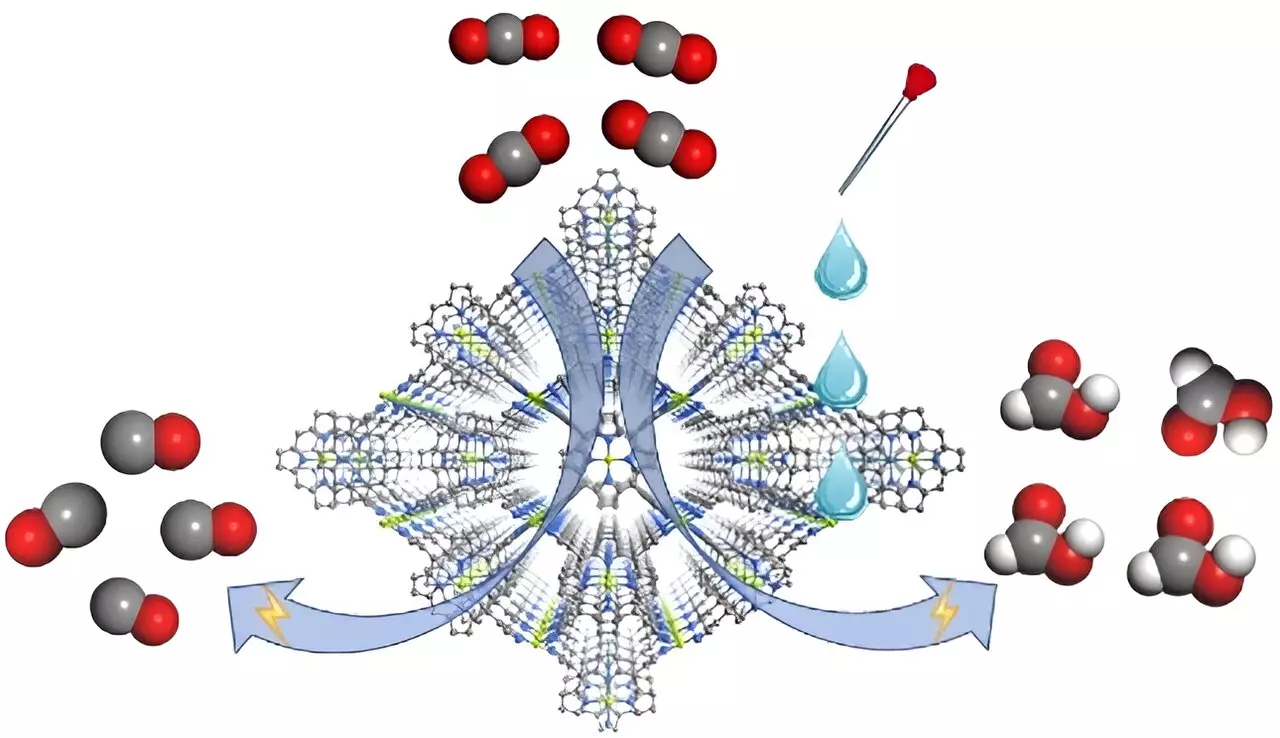
A recent study published in Angewandte Chemie International Edition has spotlighted the role of the electrolyte in tuning the selectivity of CO2 reductions. Researchers from the Fujian Institute of Research on the Structure of Matter have devised an innovative metal-organic framework (MOF) electrocatalyst named FICN-8. This catalyst, constructed from unique Cu(porphyrin)-based ligands and Cu(pyrazolate) units, showcases a three-dimensional porous architecture that facilitates high accessibility to its active sites. Such design paves the way for fine-tuning reaction conditions based on solvent and electrolyte arrangements, a factor previously overlooked in this research domain.
FICN-8 demonstrates remarkable performance, especially when paired with electrolytes like tetrabutylammonium hexafluorophosphate (TBAPF6) in acetonitrile, achieving a CO generation selectivity of 95%. Notably, the introduction of proton sources, such as water or trifluoroethanol (TFE), shifts the product profile from predominantly carbon monoxide (CO) to formic acid (HCOOH). This ability to switch outputs based on minor modifications in the electrolyte composition not only underscores the versatility of MOFs but also reveals the intricacies of the electrochemical processes involved.
To unravel the underlying mechanisms, the researchers conducted kinetic isotope effect (KIE) measurements, which provided insight into the role of protons. The KIE for CO production was almost negligible, while the value for formic acid demonstrated significant proton engagement, indicating a different pathway for CO2 reduction to HCOOH. These findings suggest that by understanding and manipulating electrolyte components, we can effectively manage the ratios of multiple products derived from CO2.
The implications of this research extend far beyond mere academic interest. The ability to engineer catalyst-electrolyte systems marks a transformative step towards developing more effective strategies for CO2 utilization. The study emphasizes the need for further exploration into electrolyte variations and their interactions with various electrocatalysts, paving the way for innovative solutions in carbon recycling.
As we venture into this newly discovered territory, the control of product selectivity via electrolyte composition could hold the key to unlocking more efficient pathways for CO2 reduction. Through continued investigation, researchers can harness and refine these interactions, ultimately contributing to sustainable development and addressing the challenges posed by climate change.