Bacteria showcase a remarkable ability to survive in harsh environments and evade host immune responses. Central to these survival strategies is their capacity to form protective structures, particularly capsular polymers that surround many bacterial pathogens. The role of these capsules in safeguarding bacteria from desiccation, physical stress, and immune detection has drawn significant interest in microbiology and pharmacology. Recent research led by Dr. Timm Fiebig at the Hannover Medical School has made strides in understanding the biochemical processes involved in capsule assembly, providing crucial insights that could pave the way for novel antibacterial therapies and vaccine development.
Capsular polymers, often composed of intricate arrangements of sugar chains, serve as a formidable barricade for bacteria. By forming a thick layer around their cell walls, these capsules obscure bacteria from immune detection, thus facilitating persistent infections. This adaptation is particularly evident in pathogens like *Haemophilus influenzae* type b (Hib), which can lead to severe diseases such as meningitis and pneumonia. The protective nature of these capsules is so vital that disrupting their formation could significantly impair bacterial survival. Consequently, understanding how these structures are synthesized is crucial not only for basic science but also for the development of effective medical interventions.
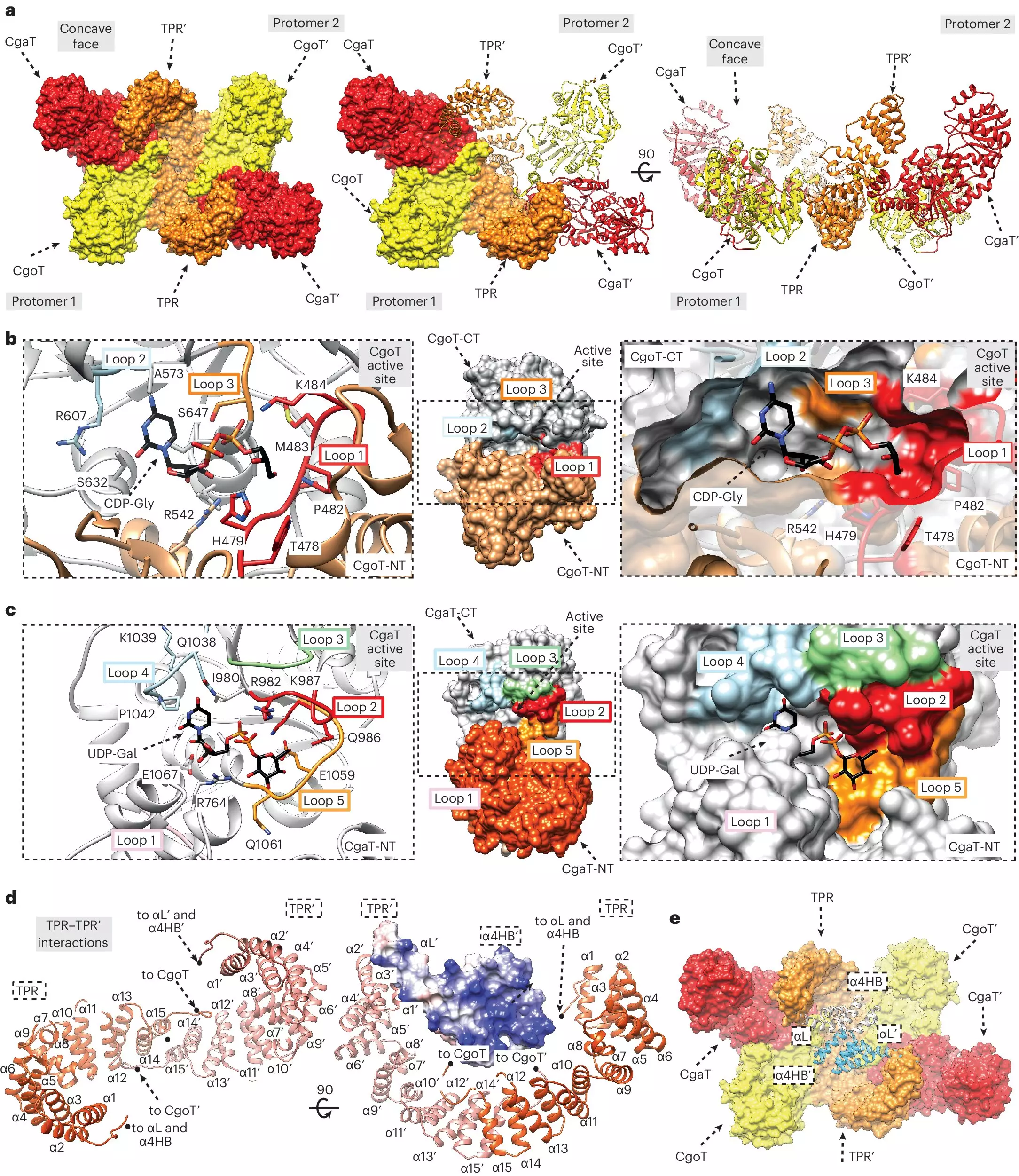
Despite the critical role capsules play in bacterial defense, how these polysaccharide chains integrate with the bacterial membrane has remained largely obscure. Recently, Dr. Fiebig’s research team identified a critical intermediate known as the linker, which connects the fatty acid molecule anchoring the capsule to the bacterial membrane. This breakthrough provides a clearer picture of the molecular architecture that supports capsule formation. Additionally, the team characterized specific enzymes, termed transition transferases, responsible for producing this linker. This identification is pivotal as it opens new avenues for drug targets aimed at crippling bacterial defenses.
Mechanistic Insights: The Role of Polymerases and Linker Enzymes
The research team made significant advances in clarifying the enzymatic processes involved in capsular biosynthesis. Transition transferases not only synthesize the linker but also interact with capsular polymerases—the enzymes responsible for creating the polysaccharide capsules themselves. By utilizing advanced chromatographic techniques, researchers could isolate these enzymes and the linker, and then replicate capsule production in vitro. Notably, the findings suggest that transition transferases might influence the length of the sugar chains produced by the polymerase, potentially enhancing the capsule’s efficacy in shielding bacteria from host immune responses.
Potential for Therapeutic Applications in Vaccines and Antibiotics
The implications of this research extend far beyond basic microbiology. By pinpointing the genetic loci of transition transferases across various bacterial genomes, the team illuminated shared pathways that could serve as targets for novel antibiotic strategies. Inactivating these enzymes may thwart the synthesis of the linker and, consequently, the capsule itself. Such a therapeutic strategy could render bacteria vulnerable to immune system attacks, effectively leveling the playing field in infections caused by organisms like Hib that use these protective structures to their advantage.
Moreover, the study’s insights into the distinct structural differences between the linker and capsular polymers challenge previous assumptions in the field. The discovery that different bacteria utilize similar mechanisms to produce anchor chains suggests a universal target for drug development that could affect multiple bacterial strains simultaneously. The potential to develop broad-spectrum antibiotics using this knowledge presents an exciting frontier in treating resistant bacterial infections.
As the field continues to evolve, the collaborative efforts demonstrated in Dr. Fiebig’s research highlight the importance of multidisciplinary approaches in tackling complex biological problems. The identification of conserved regions in bacterial genomes, along with the structural characterization of the linker, provides a compelling basis for further exploration into additional enzyme candidates involved in capsular synthesis. This knowledge not only furthers our understanding of bacterial pathogenesis but also enhances our ability to develop innovative solutions to combat infectious diseases. As researchers continue to dissect the molecular machinery of bacterial capsules, the hope is to translate these findings into effective treatments that can outpace the growing threat of antibiotic resistance.