Noble gases, long celebrated for their reluctance to engage in chemical reactions, have historically posed significant challenges for researchers seeking to understand their compounds. Despite their reputation for being inert, Neil Bartlett’s groundbreaking work over half a century ago initiated a new frontier by synthesizing the first noble gas compound, xenon hexafluoroplatinate (XePtF6). This seminal discovery marked the beginning of a journey into the complex world of noble gas chemistry, which has since seen hundreds of xenon compounds synthesized, defying expectations about the inertness of these gases.
Despite progress since Bartlett’s pivotal experiment, the study of noble gas compounds is fraught with difficulties. Most noble gas crystals are highly susceptible to moisture, complicating efforts to grow them in sufficient sizes for conventional structural analysis techniques, such as single-crystal X-ray diffraction. As a result, many of these compounds remain largely uncharacterized. Researchers have been left in the dark regarding their precise structures and the implications of their unique bonding characteristics.
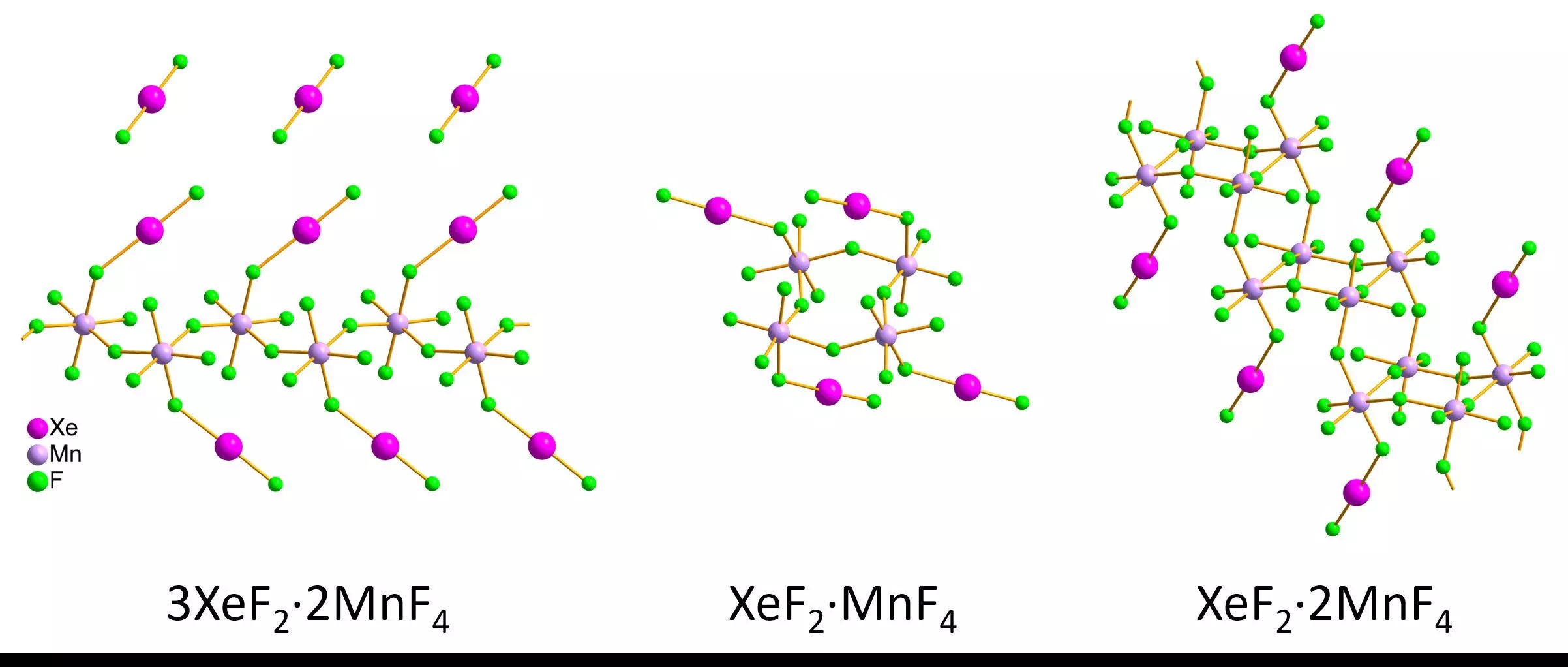
In a breakthrough, a team of researchers led by Lukáš Palatinus recently harnessed a modern technique known as 3D electron diffraction to investigate the structures of xenon-containing compounds. This technique is particularly suited to analyze small crystallites that are often resistant to moisture and can withstand exposure to air. By employing a combination of innovative sample preparation and cutting-edge microscopy, the team was able to produce and analyze nanometer-sized xenon difluoride-manganese tetrafluoride compounds, yielding valuable insights into their bond lengths and structural arrangements.
Through rigorous experimentation, the researchers synthesized various forms of xenon difluorides, including striking red crystals and pink powders. By carefully controlling conditions throughout the sample analysis, they utilized both 3D electron diffraction and single-crystal X-ray diffraction, comparing findings from the much larger micrometer-sized crystals with those obtained from the nanoscale samples. The consistency of the results significantly validates the potential of 3D electron diffraction as a reliable method for analyzing such sensitive compounds.
The implications of this study are profound. The success of 3D electron diffraction in elucidating the structures of previously elusive noble gas compounds opens the door for future research endeavors. By leveraging this technique, scientists aim to decipher the intricacies of not only the famed XePtF6 but also a variety of other noble gas compounds that have remained impervious to previous analytical methods. This work could pave the way for enhanced understanding in areas ranging from material science to potential applications in various technological innovations.
As researchers continue to advance the methods available for studying noble gases, the prospects for uncovering their secrets look brighter than ever. The marriage of innovative techniques with the intricate chemistry of noble gases will likely yield exciting discoveries that could redefine our understanding of these inert elements and their potential interactions in the chemical landscape.